Since 1991, M.TEC has been working as an engineering service provider in the medical field for well-known companies in the pharmaceuticals, diagnostics, and medical device sectors. M.TEC supports its clients from the product idea through development to realization in creating these medical applications and devices.
Our engineering teams offer interdisciplinary competencies in areas such as project management, product definition, patentability, design, development, construction, simulation, tool technology, industrialization, and PLM services. M.TEC is ISO 9001 certified, and the engineers from the high-tech region of Aachen operate according to FDA, MDR, and GMP guidelines. M.TEC has always guaranteed data security and confidentiality to its clients.
Are you looking for an engineering service provider for your medical developments?
Are you in need of a service provider that covers all development disciplines in the medical field, including the necessary documentation work? Do you want external development engineers to support your teams at every point in the engineering process, provide high-quality simulations, and possibly also offer suggestions for risk minimization and optimization? And ideally, would you also receive support in the form of creative methods and innovative techniques?
Look no further! M.TEC Engineering provides you with excellent development services and always work with the customer's best interests in mind: cost-effective, flexible, proactive, and if desired, independently. We develop and document according to our customers' quality standards and, if necessary, fully integrated into the customer's systems.
The M.TEC Development Methodology for Successful, Economical Medical Products
Our medical development experts place special emphasis on the early identification and management of risks. To this end, M.TEC employs a development methodology that questions every step in engineering from a holistic perspective.
Methodical Medical Development Process by M.TEC
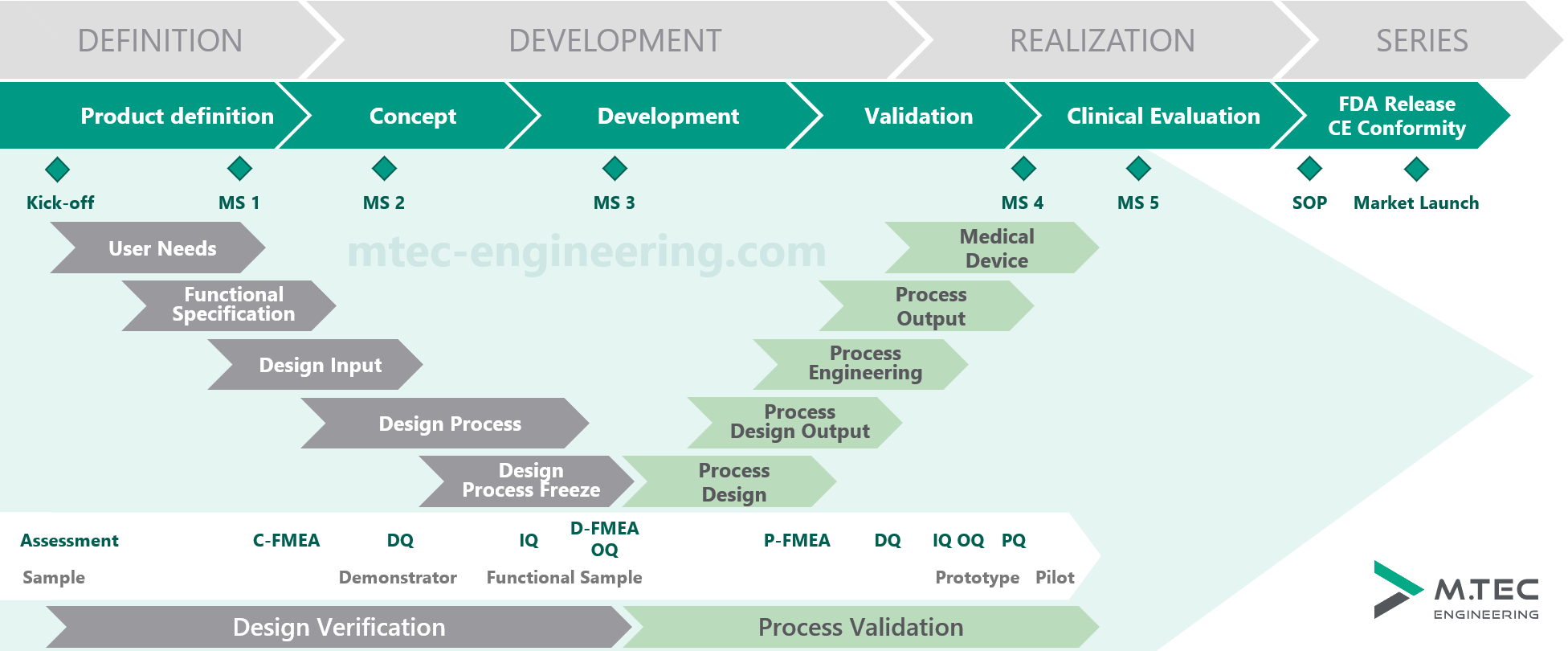
This methodology has proven to be a guarantee for a stable development process in many, sometimes long-term development contracts. The process adheres to the regulatory requirements and needs of the medical industry. Thus, M.TEC ensures a structured and comprehensible project workflow with deliberate transitions between each project phase, along with corresponding documentation.
In accordance with FDA guidelines, the process includes two overarching milestones: Design Input and Design Output, which are central phases of product development, along with additional milestones that mark the completion of subordinate sections. This process is applied in all areas of medical engineering at M.TEC.
Along the approval milestones, M.TEC not only supports its clients in development but also assists in the procurement, assembly, and testing of corresponding prototypes and functional samples (sample, demonstrator, functional sample, prototype, pilot).